
A UV bulb is sometimes referred to as a "germicidal" bulb because it inactivates germs. The bulb is similar to a fluorescent bulb, except that it lacks the internal phosphor coating and is housed in fused quartz rather than glass.
UV light is shorter wavelength (higher energy) than visible. It is called ultraviolet because it is just beyond violet light. Technically, ultraviolet light is defined to be any wavelength of light - also called electromagnetic radiation - shorter than 400 nanometer.
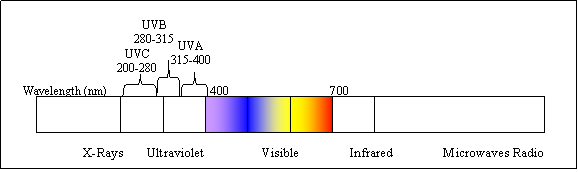
Ultraviolet light is emitted by the sun. The UVA and UVB (280-400nm) are responsible for sun tanning and sun burning. UVC, which is almost entirely filtered out through the ozone layer in the high atmosphere, can penetrate cells and cause damage to the DNA. This damage leads to the inactivation of microorganisms in drinking water.
NOTE: For the same reason that UVC is harmful to microorganisms, it can be harmful to humans as well. UVC can cause skin irritation and severe eye damage. For this reason, never look at the bulb when it is on. Direct exposure can be avoided by never opening the UV-Tube when it is plugged in to the power source, whether or not the light is on. The UV-Tube design uses a Plexiglas or glass window that completely filters out all UVC, leaving only harmless blue light. (See section on material interactions with UV light for more information on UVC penetration through plastics.)
UVC light inactivates microorganisms by damaging their genetic material (DNA) and rendering them unable to replicate.
DNA (deoxyribo nucleic acid) is essentially two intertwined strands with base pairs attached. The two strands are unwound and copied during replication of the cell. Ultraviolet light in the UVC region creates photoproducts, such as connecting adjacent base pairs, which then obstruct the replication process. With enough of these altered base pairs, replication is prevented. The maximum absorption of DNA - and maximum formation of photoproducts - occurs between 260-265 nm.
Photoproducts are formed by pyrimidine bases (thymine, cytosine and uracil) at a much higher rate than by purine bases (adenine, guanine) The most common photoproducts are cyclobutyl dimers, which are formed when two adjacent pyrimidine bases join to form a four carbon ring. Although pyrimidine dimers are often composed of two thymines, they may also be composed of two cytosines, or a thymine and a cytosine. Other, less common photoproducts include pyrimidine adducts, spore photoproducts, pyrimidine hydrates and DNA-protein crosslinks (Harm, 1980)..
UV treatment, unlike chlorination produces no known disinfection byproducts (Wolfe, 1990; National Drinking Water Clearinghouse, 2000; de Veer, 1994).
To inactivate a given microorganism the water must be hit with a certain amount or dose of UV light. Dose, more properly referred to as fluence, is determined by variables associated with the design and operation of the UV disinfection device, as well as the characteristics of the water that is treated.
Fluence is calculated as the product of light intensity at a given wavelength and exposure time. Intensity at any given point is determined by bulb strength and the geometry of the reactor. The greater the thickness of water that the light travels through, the lower the intensity received. The exposure time is governed by the geometry and the hydrodynamics of the reactor. The UV-Tube is designed such that the lowest fluence received by any of the water is sufficient to achieve the desired reduction in microorganisms. Fluence may be reported in mW-sec/cm2, which is equivalent to mJ/cm2, or in the SI units, J/m2. Standard doses delivered by a UV drinking water treatment system are between 15 and 50 mW-sec/cm2. The EPA has not finalized a rule on dose requirement.
| Units | Equivalents | |
| Energy | Joule (J) | |
| Power | Watt (W) | 1 J/s |
| Intensity | W/cm2 | 103 mW/cm2 |
| Fluence | mWs/cm2 | 1 mJ/cm2 = 103Ws/cm2 = 10J/m2 |
Intensity decreases due to attenuation and dissipation. In other words, the further from the source, the lower the strength of the light, because it is spread over a larger area (due to dissipation) and the lower the strength of the light, because it interacts with molecules in the water (attenuation). While dissipation is predicted simply by the geometry of the UV-Tube, attenuation depends on characteristics of the water. If the water contains a high concentration of materials that absorb UV light, then less UV will be transmitted. The amount of light absorbed per centimeter is expressed as the absorption coefficient (a). As this coefficient increases, transmissivity decreases exponentially, therefore the absorption coefficient of the water plays a very important role in the effectiveness of the UV disinfection device.
The absorption coefficient (a) describes how much light is lost as it travels through a medium. It can be determined experimentally and is reported in inverse centimeters. The absorption coefficient of pure distilled water is close to zero. Natural organic matter, iron, nitrate and manganese absorb UVC light and will increase the absorption coefficient of a water sample (Kolch, 1999). Absorption coefficients in drinking water would be expected in the range of 0.01 to 0.2 cm-1. The Absorbance of 7 drinking water samples taken around the Pátzcuaro region were in the range of 0.002 to 0.009cm-1, which correspond to a naperian absorption coefficient (base e, represented by the symbol alpha) between 0.005 and 0.021 cm-1.One sample, taken from a muddy puddle, had an Absorbance of 0.048, or a naperian absorption coefficient of 0.111 cm-1. According to Snicer et al., water with a naperian absorption coefficient of 0.125 cm-1would be considered of fair water quality (, 1997).
Just as the recommended dose has not been standardized, neither have allowable levels of UV absorbing compounds. However, maximum concentrations of iron and manganese have been recommended at 0.03 mg/l and 0.02 mg/l, respectively (Kolch, 1999) and turbidity less than 5 NTU (Bolton, 2001). The issue of turbidity and particulate concentration is discussed below.
The higher the flow rate, the shorter the hydraulic detention time, therefore the smaller the dose received by the water. An appropriate flow rate must be determined based on the other characteristics of the water and on the desired dose.
Turbidity is a measure of the quantity of particulates in a solution. It is determined by shining an infrared beam of light through a one centimeter thick sample and measuring light detected by sensors placed at ninety degrees to the beam. Turbidity is not necessarily correlated with the absorption coefficient. Turbidity is commonly reported in NTU.
Turbidity is often thought to be a limiting feature in ultraviolet disinfection. However, work has shown that particles, as long as they are not UV-absorbers, do not significantly reduce the overall irradiance by either shading or scattering, but only when organisms are embedded within them (Linden, 1998; Emerick, 1999). Particle suspension can increase the apparent absorption coefficient - as measured by a spectrophotometer - by scattering rather than absorbing light (Linden, 1998). This effect can lead to under prediction of design capabilities. In the US this would only be a concern in wastewater treatment, however in developing country applications high particle concentration may be relevant in drinking water as well.
For a given sized UV-Tube, determining the best water depth (or weir height) involves a tradeoff between residence time and water thickness. The higher the water height, the greater the volume of water in the tube at any given flow rate, and therefore the greater the average residence time. However, the higher the water height, the greater the attenuation of light and the lower the dose reaching the water at the very bottom of the tube. Since attenuation is proportional to the absorption coefficient, the optimal water height will depend on the absorption coefficient. The optimal weir height (or water depth) is inversely related to the absorption coefficient.
The maximum UV absorbance of DNA, 260-265 nm, coincides well with peak output of low pressure mercury arc lamps at 253.7 nm. Two different types of lamps are typically used in water disinfection, medium pressure and low pressure mercury vapor arc lamp. The table below displays these differences.
| Characteristic | Low Pressure / Low Intensity | Medium Pressure / High Intensity |
| Typical Energy Use: | 60 W | 5,000 W |
| Percentage Output at 253.7 nm: | 88% | 44% |
| Ozone Production: | NONE | POSSIBLE (quartz can be doped to prevent formation) |
| Susceptibility to Cooling: | YES | NO |
| Susceptibility to Cooling: | GOOD | POOR |
| Benefits: | EFFICIENCY (lower energy requirements) | SMALLER, LESS MAINTENANCE, USE WITH POOR QUALITY WATER |
Current UV-Tube designs use a low pressure mercury vapor arc lamp.
Fluence depends on bulb strength. A 30 Watt low pressure GE T8 bulb emits approximately 5 Watts at 254 nm. As the bulb ages its strength will slowly diminish. GE recommends a replacement after 7,500 hours, assuming bulb is turned on for 3 hour periods. Each start is expected to decrease the bulbs lifetime, so if the bulb is left on for periods greater than 3 hours, it may last longer. Conversely, if a bulb is turned on and off for periods shorter than 3 hours, its lifetime may be reduced to fewer than 7,500 hours. There is a need for more information on the effect of bulb on/off cycles, temperature conditions and general deteriation over time. The UV-Tube Project is currently testing bulb performance to answer some of these questions and provide better guidance on appropriate UV-Tube operation to users.